All liquid food that we consume primarily consists of water. For example, in the case of milk, 88% of its composition is water. Although milk contains various substances, its main component is water, and it is not considered a solution because these components are not uniformly mixed. Substances that are not evenly mixed and appear cloudy are called colloids. The particles within colloids are very small and do not settle over time, remaining in a continuous opaque state. When substances mixed in a solvent are larger than micrometers (μm) and settle over time, it is called a suspension. Unfiltered orange juice is a suspension, which is why it needs to be shaken well before drinking. Therefore, the particles in unfiltered orange juice settle over time, separating into a clear top layer and a cloudy bottom layer.
Colloids like milk exhibit important properties of water. As previously explained, water is a polar molecule with strong hydrogen bonding interactions between its molecules. To dissolve in such water, a foreign substance must break the hydrogen bonds between water molecules and fit in between them. For this foreign substance to be accepted by water, it must not incur a disadvantage compared to when there was only water. Water would be more accommodating to a new friend if water molecules interact with foreign substances more favorably than water molecules do with their own molecules (i.e., water molecules). To befriend water, the foreign substance needs to provide more significant interactions than just hydrogen bonds. It must form stronger interactions or more hydrogen bonds. Such substances need to have polarity. For a substance to have strong polarity, it should consist of small molecules and be composed of elements that have a “liking for electrons” as part of their nature. Salt is an example of an ionic compound, made up of positively charged sodium ions (Na+) and negatively charged chloride ions (Cl–). When it dissolves in water, sodium willingly gives its electron to chlorine, resulting in sodium becoming a cation and chloride anion, allowing them to engage in “ion-dipole interactions” with water’s oxygen and hydrogen atoms, respectively. When molecules interact at this level, we call it a homogeneous mixture. Sugar dissolves in water without breaking molecules down because it contains many hydroxyl (OH) groups with polarity. Each hydroxyl group forms hydrogen bonds with several water molecules. Again, interactions occur at the molecular level.
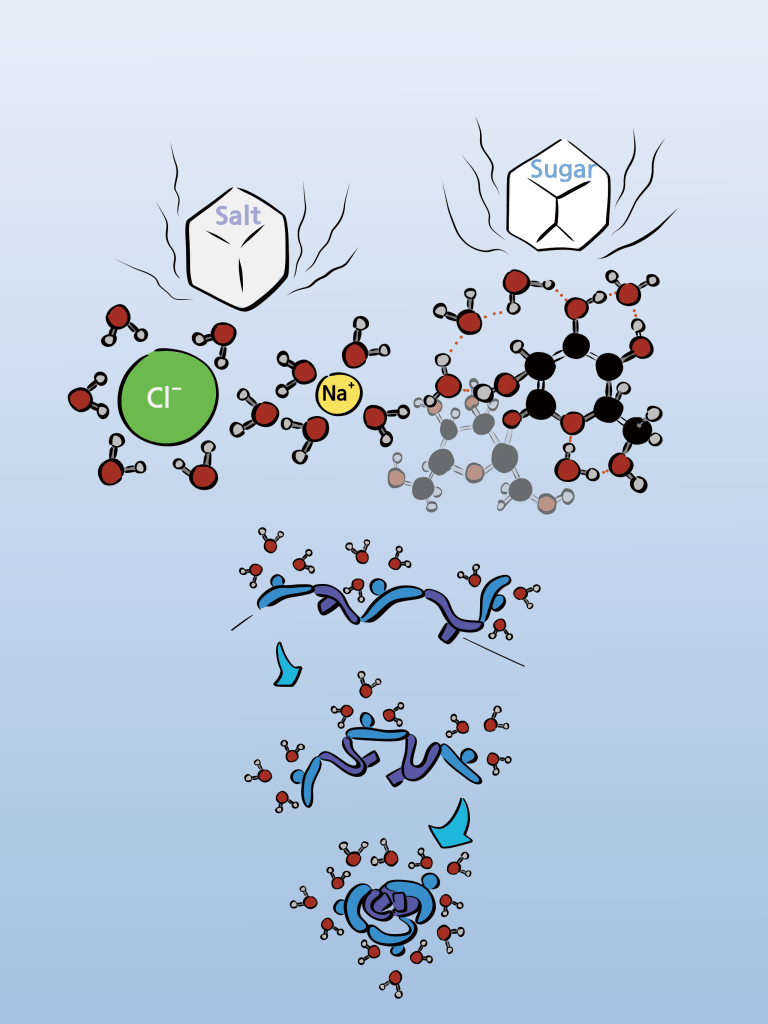
Non-polar molecules with low polarity, like lipids, do not interact with water molecules. They cannot form bonds with each other and are thus rejected by the water molecules. This is why, for example, when you add salt to oil, it doesn’t dissolve but rather settles at the bottom. When these non-polar substances become prevalent in water, colloids like milk can form (although some physical assistance may be required). From this, we can understand an important chemical principle: depending on the significant property of molecules, polarity, a solution may or may not form. The first law of chemical interactions, which can be interpreted as ‘like dissolves like,’ is something we experience every day in the beverages we consume.
However, just as the world cannot be explained solely through black-and-white logic like in comics or movies (even though modern comics and movies don’t always follow this pattern), It is not always possible to classify substances found in nature as either polar or non-polar molecules, as they can exhibit a variety of characteristics that make them difficult to categorize. Many molecules have both polar and non-polar groups, and some molecules have such high polarity that they do not interact with water (We refer to it as polar hydrophobicity.). The three major nutrients—carbohydrates, proteins, and fats—exhibit this complexity. All three are organic compounds based on carbon. Hydrocarbons, which are the simplest organic compounds composed of carbon and hydrogen, are non-polar molecules. Nutrient molecules are composed primarily of hydrocarbons, with the incorporation of other elements such as phosphorus (P), oxygen (O), nitrogen (N), and sulfur (S), to form polar groups. These molecules contain a combination of polar and non-polar groups. The polar groups interact directly with water while the non-polar groups do not. As a result, they perform complex functions in water, forming three-dimensional structures and playing crucial roles in various life processes in our bodies.
More than 60% of our bodies consist of water. The mysterious solvent properties of water allow the diverse functions of the nutrients that make up our bodies, creating an optimal environment for vital life processes. From a chemist’s perspective, if we were to look at cells, they are cities covered in water where proteins interact with lipids, carbohydrates, and each other. At the molecular level, water enables a wide range of interactions, making it an essential nutrient. It enables life processes and allows us to create and enjoy flavorful and diverse foods.
References
1. Lucy and Harris, (2017) Quantitative Chemical Analysis. 9th Ed., W.H. Freeman and Co.
2. Oxtoby et al. (2002) Principles of modern chemistry. Thomson/Brooks/Cole
3. World Health Organization. Sustainable Development and Healthy Environments Cluster. (2005). Nutrients in drinking water. World Health Organization.
4. Sengupta, Int. J.

Leave a reply to Meat, Meat, Meat! – Leo & Leah Cancel reply