All Beverages are Aqueous Solutions
- Solution: A substance in which two or more materials are uniformly mixed.
- A solution is composed of a solvent and a solute.
- The substance in larger quantity is called the solvent, and the substance in smaller quantity is called the solute.
- An aqueous solution is a solution where water is the solvent.
• Any substance that can dissolve in water can be a solute.
• Juices, tea, carbonated drinks, ion drinks, alcohol, etc., all beverages are aqueous solutions.
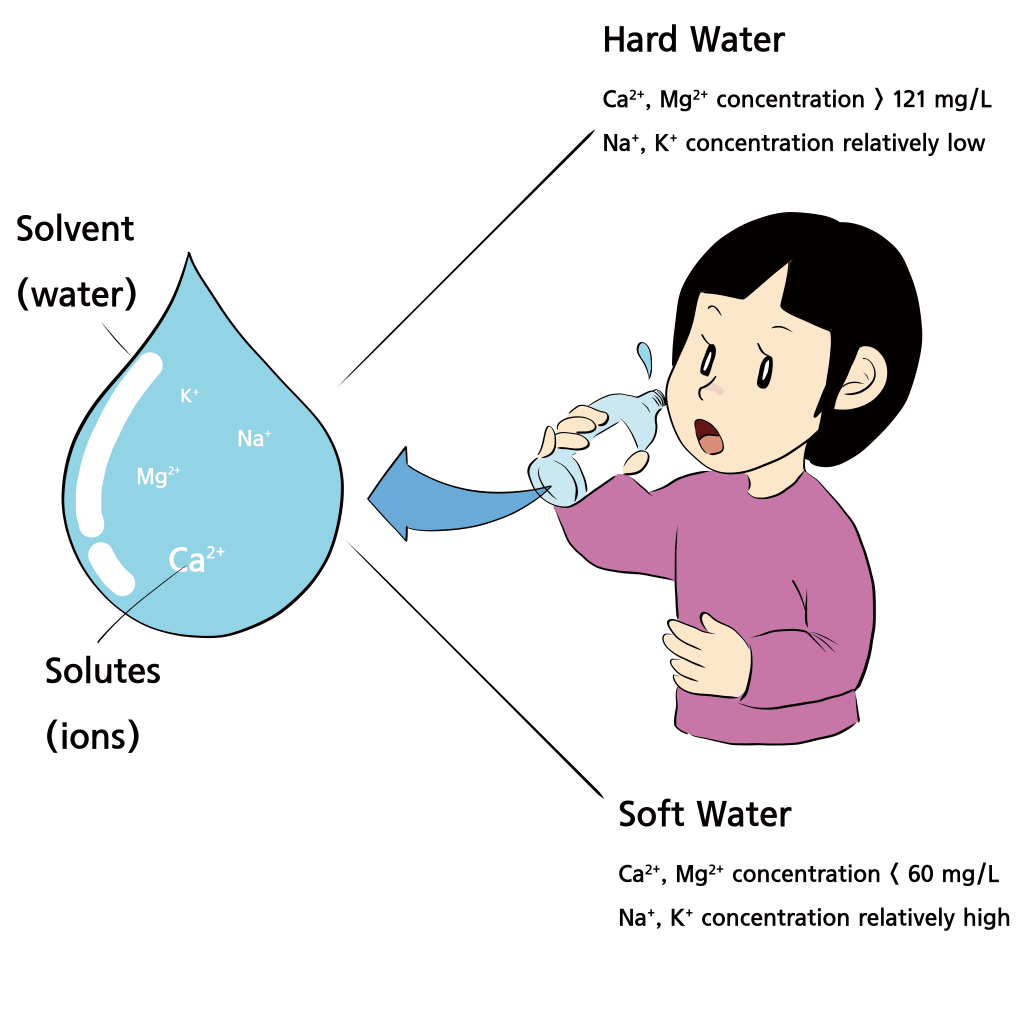
- The type and amount of minerals in water, as well as its acidity, determine the taste of the water.
- It is said that water tastes best when the total mineral content is 100mg/L.
- Hard water (turbid): High content of calcium and magnesium ions (> 120mg/L), Turbid and bitter taste
- Soft water: High content of sodium and potassium ions
- pH 7 neutral: tasteless, pH 5 – 6.7 acidic: sour taste, pH 7.8 – 10 basic: bitter taste
- Europe has a lot of limestone in its soil, which produces hard water.
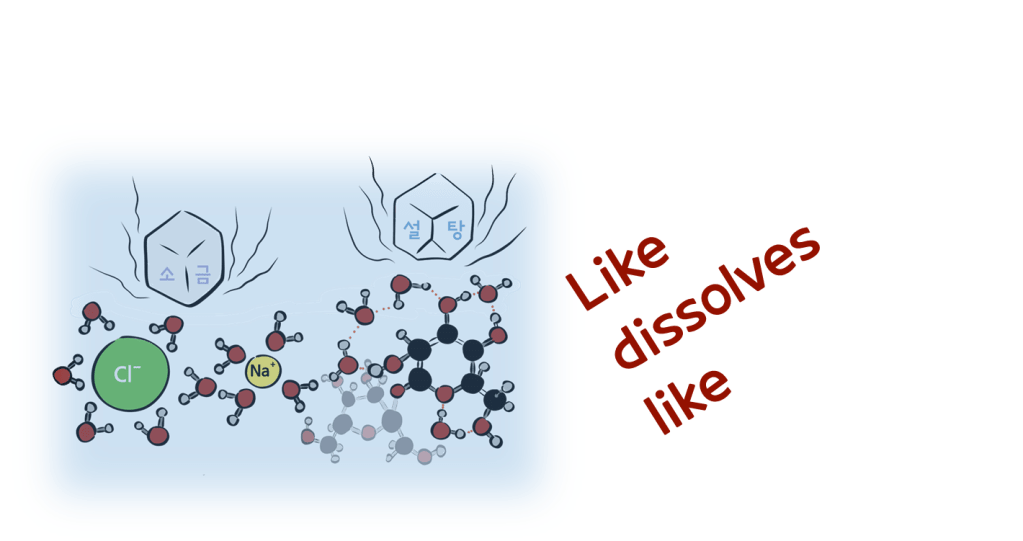
- A solute can be any substance that dissolves in water.
- However, only substances that have a charge like ions (i.e. salt) or possess polar functional groups (i.e. sugar) can dissolve in water.
- Only polar substances dissolve in polar solvents like water.
- The first rule of this chemical interaction, which can be interpreted as “Like dissolves like,” is something we experience every day with the beverages we drink.
Water, the Basis of Life Phenomena

- Natural substances can’t be classified solely as hydrophilic or hydrophobic.
- Many molecules have both types of functional groups.
- Examples include carbohydrates, proteins, and fats.
- Proteins have hydrophilic groups that interact with water and hydrophobic groups that don’t.
- This creates 3D protein structures in water, essential for various body functions.
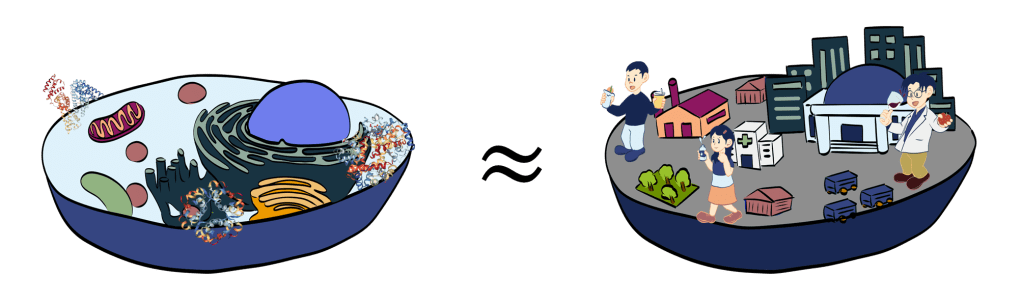
- Water’s solvent properties allow nutrients to perform various functions, making it essential for life.
- Cities are covered by air, where humans, animals, and plants interact.
- Cells are like cities where proteins interact with lipids and carbohydrates.

Leave a comment