Coffee and tea are fundamentally very similar beverages. They are aqueous solutions made by extracting organic and inorganic substances contained in coffee beans and tea leaves using hot water. Both tea and coffee contain various aromatic compounds. Aromatic compounds are substances that have a scent, are generally volatile, and can easily be detected by the olfactory system. In both coffee and tea, caffeine is the main active ingredient. The unique aroma and caffeine content are likely reasons why coffee and tea have long been beloved as representative beverages by many people.
Processed coffee beans contain hundreds of compounds, with about several dozen of them determining the aroma of the coffee. The molecules that determine the aroma of coffee are formed during the roasting process. Originally, coffee beans are yellow-green in color. The process of roasting involves turning these yellow-green beans into brown beans.
The first chemical reaction that occurs during the roasting process is called the Maillard reaction. The Maillard reaction is a chemical reaction that occurs between carbohydrates and amino acids in food when exposed to heat (300-390°F). This reaction forms a pigment called melanoidin, which gives the food a brown color. The browning effect due to the Maillard reaction is commonly observed when heating carbohydrate-rich foods such as bread, cookies, and popcorn. Besides melanoidin, hundreds of different compounds can be formed during the Maillard reaction. Among these compounds, 2-furfurythiol (fufury mercaptan) is the primary contributor to the characteristic smell of coffee beans. Another substance formed is trigonelline, which is a major cause of coffee’s bitter taste. Along with the Maillard reaction, caramelization also occurs. At temperatures of 338 – 392°F (150 – 200°C), the carbohydrate components undergo thermal decomposition and form polymers. During the pyrolysis process, various molecules are formed, among which diacetyl gives the characteristic caramel flavor. Although browning also occurs during caramelization, it is a different chemical reaction from the Maillard reaction where amino acids and carbohydrates react.
When the roasting temperature exceeds approximately 392°F (200°C), the water inside the coffee beans evaporates, causing the beans to crack and become brittle (1st crack). During this process, the size of the beans nearly doubles. As the temperature reaches around 428°F (220°C), various organic substances in the coffee oxidize and are released as carbon dioxide. At about 437°F (225°C), the cellulose fibers in the coffee beans decompose, and aromatic substances called caffeol are released (2nd crack). These aromatic organic substances make the coffee beans oily. This organic material forms the crema, the foam layer on the surface of an espresso extracted from roasted coffee beans. Roasting techniques have evolved to control the type and amount of aromatic compounds produced by adjusting the type of beans, heating time, and temperature. Over time, the process of producing coffee beans with various aromas through controlled roasting has developed into an independent cultural and industrial sector.
Among the components of coffee, chlorogenic acid is a polyphenolic compound with antioxidant properties that can help prevent a rapid rise in blood sugar levels after meals. A small size cup of coffee (6 – 8 oz) contains 70 – 350 mg of chlorogenic acid. Various studies report that the chlorogenic acid in coffee may help prevent type 2 diabetes, Parkinson’s disease, and liver diseases. However, the longer the roasting process, the more chlorogenic acid is thermally decomposed and lost. Therefore, dark roast coffee contains very low levels of chlorogenic acid.
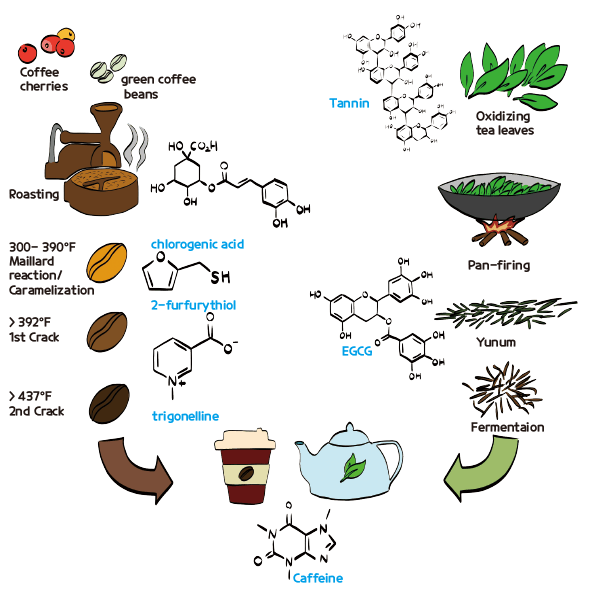
There are many different types of tea in the world. It is not an exaggeration to say that tea has a longer history and has formed a larger culture compared to coffee. Generally, “tea” refers to a beverage made by steeping the leaves of the tea plant. Green tea, black tea, oolong tea, white tea, and pu-erh tea all come from the same tea plant leaves. Green tea and black tea are named after the color of the tea when brewed with hot water. The reason different teas are made from the same tea leaves, but have different colors and aromas, is due to differences in the manufacturing process. Of course, the tea plant’s growing region, leaf size, and harvest time also have an impact.
Tea is basically made through processes of drying, oxidizing, stopping oxidation by roasting or steaming, and fermenting the tea leaves. The main difference between green tea and black tea is the degree of oxidation of the tannin components in the tea leaves. Green tea is oxidized by about 10%, while black tea is oxidized by about 85%. Tannin is a polyphenol that gives an astringent taste and is synthesized in plants. Polyphenolic molecules have antioxidant properties. Antioxidant activity refers to the reaction of readily oxidizable molecules with reactive oxygen species or other oxidizing agents, thereby preventing oxidation of surrounding substances. Therefore, antioxidant substances prevent the oxidation of surrounding substances until they are fully oxidized and depleted. A representative antioxidant substance contained in tea leaves is epigallocatechin gallate (EGCG). The process of oxidizing tea leaves is also referred to as “fermentation,” which is a misnomer but is commonly used. When tannins are oxidized, the astringent taste diminishes, and the color turns yellow or red.
The process of rolling and bruising tea leaves, known as “yunum,” is intended to create small wounds on the leaves. By physically breaking down the cell walls of the tea leaves, their components are released. The process of steaming or pan-firing the tea is similar in purpose to coffee roasting but slightly different. The main goal is to prevent the oxidation of the tea leaves by denaturing the enzyme polyphenol oxidase, which is responsible for oxidation. Additionally, this process evaporates moisture from the tea leaves, allowing them to be stored for longer periods. The evaporation of moisture and the release of aromatic molecules in the tea leaves are similar to the roasting process of coffee.
In the case of pu-erh tea, there is an additional microbial fermentation process (post-fermentation) after the steaming or pan-firing process. It is stored in a high-humidity environment to allow fermentation by a mold called Aspergillus niger. Tea leaves contain thousands of compounds, and through processing, these compounds break down and combine to form numerous other compounds. This is why the same type of tea can have different aromas and flavors depending on the region and processing method.
Caffeine is a common active ingredient found in both coffee and tea. Generally, coffee contains slightly more caffeine than tea. One cup (8 oz) of green tea contains 28 mg of caffeine, while a cup of black tea contains 47 mg, which is less than half the amount in a cup of filtered coffee (96 mg of caffeine). Espresso coffee, using high temperature and pressure to efficiently extract components from the beans with a small amount of water, contains 64 mg of caffeine in a 30mL shot. Compared to the caffeine content in a can of cola (25 mg per 8 oz), tea and coffee have significantly higher amounts of caffeine. While moderate consumption is fine, it is important to note that both coffee and tea can be addictive if consumed in excess.
References
- Belitz et al. (2009) ‘Aroma Substances’ in Food Chemistry, 4th Ed., Springer
- Ochiai et al. J. Chromatogr, 2014, 1371, 65
- ACS Reactions, Why Does Your Coffee Taste and Smell Delicious?
- Wang and Yao, Food Chem., 2011, 128, 573
- https://pubchem.ncbi.nlm.nih.gov/compound/Chlorogenic-acid
- Moon et al. J. Agric. Food Chem. 2009, 57, 12, 5365
- https://www.teaclass.com
- https://www.teaguardian.com/what-is-tea/green-tea-production-roasting
- http://www.tocklai.org/activities/tea-chemistry

Leave a comment